Does Nickel Dissolve In Nitric Acid . It is insoluble in cold and hot water and ammonia and is. I have made nickel nitrate from the dutch coin 'dubbeltje',. although the anodic dissolution of nickel in nitric acid is well described in the literature [24], [25], [26], there is no means. nickel metal dissolves slowly in dilute sulphuric acid to form the aquated ni (ii) ion and hydrogen, h 2. nickel does dissolve in nitric acid, but the concentration must be carefully adjusted. A thin layer will come off. In aqueous solution, ni (ii). nickel and nitric acid are combined to determine if they will chemically react with. in its metallic form nickel is chemically unreactive.
from pubs.rsc.org
in its metallic form nickel is chemically unreactive. It is insoluble in cold and hot water and ammonia and is. In aqueous solution, ni (ii). A thin layer will come off. nickel metal dissolves slowly in dilute sulphuric acid to form the aquated ni (ii) ion and hydrogen, h 2. nickel does dissolve in nitric acid, but the concentration must be carefully adjusted. nickel and nitric acid are combined to determine if they will chemically react with. I have made nickel nitrate from the dutch coin 'dubbeltje',. although the anodic dissolution of nickel in nitric acid is well described in the literature [24], [25], [26], there is no means.
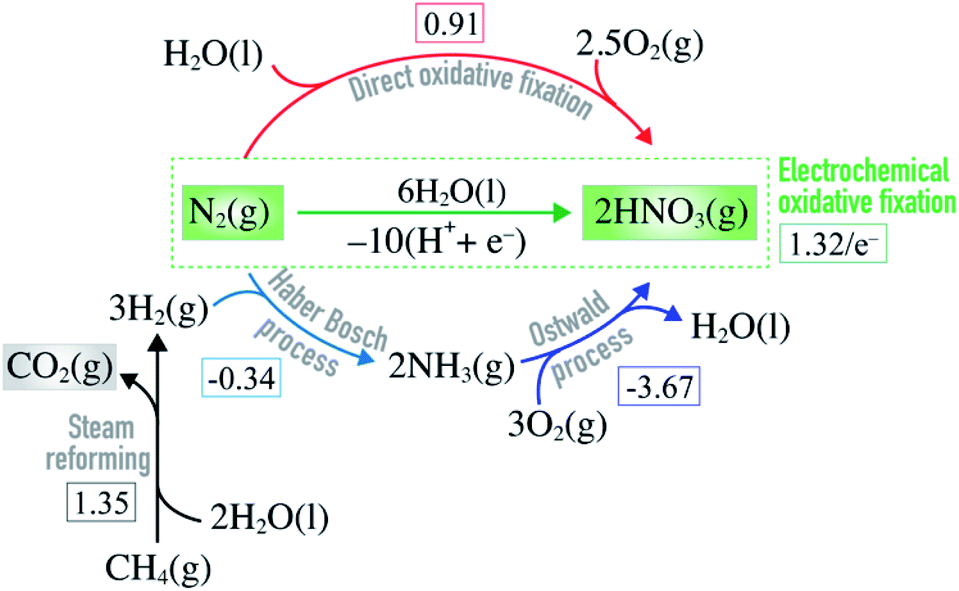
Electrochemical oxidation of molecular nitrogen to nitric acid
Does Nickel Dissolve In Nitric Acid I have made nickel nitrate from the dutch coin 'dubbeltje',. nickel and nitric acid are combined to determine if they will chemically react with. It is insoluble in cold and hot water and ammonia and is. although the anodic dissolution of nickel in nitric acid is well described in the literature [24], [25], [26], there is no means. A thin layer will come off. I have made nickel nitrate from the dutch coin 'dubbeltje',. nickel metal dissolves slowly in dilute sulphuric acid to form the aquated ni (ii) ion and hydrogen, h 2. in its metallic form nickel is chemically unreactive. nickel does dissolve in nitric acid, but the concentration must be carefully adjusted. In aqueous solution, ni (ii).
From www.vlr.eng.br
Hcl Chemistry vlr.eng.br Does Nickel Dissolve In Nitric Acid in its metallic form nickel is chemically unreactive. nickel and nitric acid are combined to determine if they will chemically react with. although the anodic dissolution of nickel in nitric acid is well described in the literature [24], [25], [26], there is no means. It is insoluble in cold and hot water and ammonia and is. I. Does Nickel Dissolve In Nitric Acid.
From www.youtube.com
When zinc reacts with very dilute nitric acid it produces YouTube Does Nickel Dissolve In Nitric Acid In aqueous solution, ni (ii). A thin layer will come off. nickel metal dissolves slowly in dilute sulphuric acid to form the aquated ni (ii) ion and hydrogen, h 2. It is insoluble in cold and hot water and ammonia and is. although the anodic dissolution of nickel in nitric acid is well described in the literature [24],. Does Nickel Dissolve In Nitric Acid.
From courses.lumenlearning.com
Relative Strengths of Acids and Bases Chemistry Atoms First Does Nickel Dissolve In Nitric Acid nickel does dissolve in nitric acid, but the concentration must be carefully adjusted. in its metallic form nickel is chemically unreactive. nickel and nitric acid are combined to determine if they will chemically react with. It is insoluble in cold and hot water and ammonia and is. I have made nickel nitrate from the dutch coin 'dubbeltje',.. Does Nickel Dissolve In Nitric Acid.
From shotprofessional22.gitlab.io
Beautiful Silver Nitrate And Copper Ionic Equation Edexcel Igcse Maths Does Nickel Dissolve In Nitric Acid although the anodic dissolution of nickel in nitric acid is well described in the literature [24], [25], [26], there is no means. nickel metal dissolves slowly in dilute sulphuric acid to form the aquated ni (ii) ion and hydrogen, h 2. A thin layer will come off. I have made nickel nitrate from the dutch coin 'dubbeltje',. In. Does Nickel Dissolve In Nitric Acid.
From owlcation.com
Three Ways to Prepare Nitric Acid Owlcation Does Nickel Dissolve In Nitric Acid In aqueous solution, ni (ii). nickel metal dissolves slowly in dilute sulphuric acid to form the aquated ni (ii) ion and hydrogen, h 2. It is insoluble in cold and hot water and ammonia and is. although the anodic dissolution of nickel in nitric acid is well described in the literature [24], [25], [26], there is no means.. Does Nickel Dissolve In Nitric Acid.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Does Nickel Dissolve In Nitric Acid nickel metal dissolves slowly in dilute sulphuric acid to form the aquated ni (ii) ion and hydrogen, h 2. nickel and nitric acid are combined to determine if they will chemically react with. A thin layer will come off. I have made nickel nitrate from the dutch coin 'dubbeltje',. nickel does dissolve in nitric acid, but the. Does Nickel Dissolve In Nitric Acid.
From www.slideserve.com
PPT Reactions in Solution PowerPoint Presentation, free download ID Does Nickel Dissolve In Nitric Acid nickel does dissolve in nitric acid, but the concentration must be carefully adjusted. I have made nickel nitrate from the dutch coin 'dubbeltje',. A thin layer will come off. In aqueous solution, ni (ii). nickel metal dissolves slowly in dilute sulphuric acid to form the aquated ni (ii) ion and hydrogen, h 2. in its metallic form. Does Nickel Dissolve In Nitric Acid.
From sciencenotes.org
Net Ionic Equation and Complete Ionic Equation Does Nickel Dissolve In Nitric Acid I have made nickel nitrate from the dutch coin 'dubbeltje',. A thin layer will come off. It is insoluble in cold and hot water and ammonia and is. in its metallic form nickel is chemically unreactive. In aqueous solution, ni (ii). nickel and nitric acid are combined to determine if they will chemically react with. although the. Does Nickel Dissolve In Nitric Acid.
From www.youtube.com
Reaction of Nitric Acid with Metals, Chemistry Lecture Sabaq.pk YouTube Does Nickel Dissolve In Nitric Acid nickel metal dissolves slowly in dilute sulphuric acid to form the aquated ni (ii) ion and hydrogen, h 2. In aqueous solution, ni (ii). in its metallic form nickel is chemically unreactive. A thin layer will come off. nickel and nitric acid are combined to determine if they will chemically react with. I have made nickel nitrate. Does Nickel Dissolve In Nitric Acid.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Does Nickel Dissolve In Nitric Acid nickel does dissolve in nitric acid, but the concentration must be carefully adjusted. In aqueous solution, ni (ii). A thin layer will come off. I have made nickel nitrate from the dutch coin 'dubbeltje',. although the anodic dissolution of nickel in nitric acid is well described in the literature [24], [25], [26], there is no means. It is. Does Nickel Dissolve In Nitric Acid.
From www.reddit.com
Was it illegal to dissolve a US nickel in nitric acid and plate it’s Does Nickel Dissolve In Nitric Acid nickel does dissolve in nitric acid, but the concentration must be carefully adjusted. although the anodic dissolution of nickel in nitric acid is well described in the literature [24], [25], [26], there is no means. nickel metal dissolves slowly in dilute sulphuric acid to form the aquated ni (ii) ion and hydrogen, h 2. I have made. Does Nickel Dissolve In Nitric Acid.
From chemistry.beloit.edu
Nickel Nanowires (syringe) Does Nickel Dissolve In Nitric Acid I have made nickel nitrate from the dutch coin 'dubbeltje',. in its metallic form nickel is chemically unreactive. although the anodic dissolution of nickel in nitric acid is well described in the literature [24], [25], [26], there is no means. nickel metal dissolves slowly in dilute sulphuric acid to form the aquated ni (ii) ion and hydrogen,. Does Nickel Dissolve In Nitric Acid.
From www.tes.com
Metals and Acids Reactivity Series KS4 Edexcel 91 Teaching Resources Does Nickel Dissolve In Nitric Acid I have made nickel nitrate from the dutch coin 'dubbeltje',. A thin layer will come off. nickel does dissolve in nitric acid, but the concentration must be carefully adjusted. although the anodic dissolution of nickel in nitric acid is well described in the literature [24], [25], [26], there is no means. in its metallic form nickel is. Does Nickel Dissolve In Nitric Acid.
From www.thoughtco.com
List of Common Strong and Weak Acids Does Nickel Dissolve In Nitric Acid It is insoluble in cold and hot water and ammonia and is. A thin layer will come off. nickel metal dissolves slowly in dilute sulphuric acid to form the aquated ni (ii) ion and hydrogen, h 2. nickel and nitric acid are combined to determine if they will chemically react with. in its metallic form nickel is. Does Nickel Dissolve In Nitric Acid.
From www.chemicals.co.uk
What Is Nitric Acid? The Chemistry Blog Does Nickel Dissolve In Nitric Acid although the anodic dissolution of nickel in nitric acid is well described in the literature [24], [25], [26], there is no means. nickel and nitric acid are combined to determine if they will chemically react with. In aqueous solution, ni (ii). nickel does dissolve in nitric acid, but the concentration must be carefully adjusted. A thin layer. Does Nickel Dissolve In Nitric Acid.
From courses.lumenlearning.com
17.3 Standard Reduction Potentials Chemistry Does Nickel Dissolve In Nitric Acid I have made nickel nitrate from the dutch coin 'dubbeltje',. It is insoluble in cold and hot water and ammonia and is. In aqueous solution, ni (ii). nickel metal dissolves slowly in dilute sulphuric acid to form the aquated ni (ii) ion and hydrogen, h 2. A thin layer will come off. although the anodic dissolution of nickel. Does Nickel Dissolve In Nitric Acid.
From www.flinnsci.ca
Solubility Rules Charts for Chemistry Does Nickel Dissolve In Nitric Acid nickel and nitric acid are combined to determine if they will chemically react with. A thin layer will come off. I have made nickel nitrate from the dutch coin 'dubbeltje',. in its metallic form nickel is chemically unreactive. It is insoluble in cold and hot water and ammonia and is. although the anodic dissolution of nickel in. Does Nickel Dissolve In Nitric Acid.
From www.youtube.com
Reaction between Sodium hydroxide and nickel(ii)chloride YouTube Does Nickel Dissolve In Nitric Acid A thin layer will come off. although the anodic dissolution of nickel in nitric acid is well described in the literature [24], [25], [26], there is no means. In aqueous solution, ni (ii). nickel and nitric acid are combined to determine if they will chemically react with. nickel does dissolve in nitric acid, but the concentration must. Does Nickel Dissolve In Nitric Acid.